Colin Bailie, Stanford University
Maximizing the efficiency of converting sunlight to electricity was the primary goal for much of the history of solar power industry. Because solar cells were so expensive to make, they were used only in special applications, such as on spacecraft, where performance was more important than cost. That game changed a couple decades ago with the advent of thin-film solar cells that forced the industry to focus on lower costs rather than high performance.
Now that solar cells are less expensive to manufacture, the industry has entered a third phase with the goal: increasing efficiency while keeping low-cost manufacturing.
Most commercial solar photovoltaic cells are made from silicon. To push the efficiency higher, one of the best options is to make tandem solar cells – that is, cells that use multiple light-absorbing materials. For perspective, silicon solar cells have a record efficiency of 25.6%. Using one light-absorbing material, the theoretical limit is 34% efficiency. Using two light-absorbing materials in tandem pushes the theoretical limit to 46% efficiency.
My colleagues and I made tandem solar cells from two light-absorbing materials: silicon and the metal-halide perovskite, a new material with the potential to be manufactured at low cost. In a paper published this week, we showed how these two materials can be connected in a single solar cell and a way to harvest the power in a novel way.
These developments lay the foundation for silicon-perovskite tandem solar cells and may provide a path forward for the solar industry to make high-efficiency, low-cost solar cells.
Capturing more of the light
One way to reduce the cost of solar is to improve the efficiency of the solar panels. With a higher efficiency, fewer panels, or modules, need to be installed to reach a desired power target. This means less labor, less land and less hardware.
To understand why a tandem cell offers a boost in efficiency, one has to look at how different solar cell materials react to incoming light.
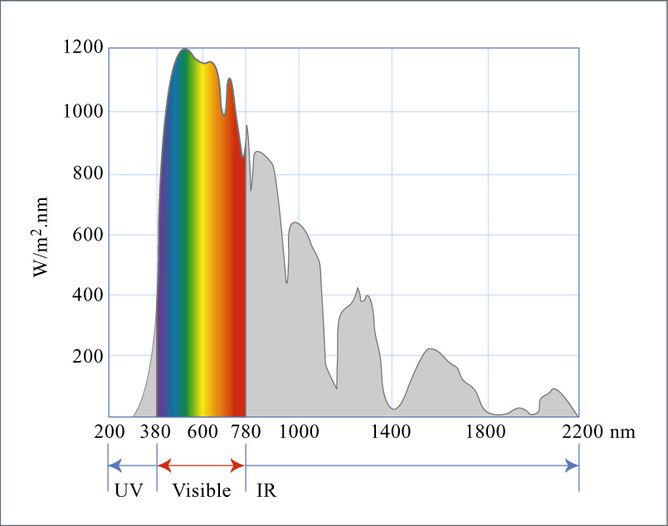
Sunlight is made up of a wide variety of energies, from ultraviolet light and visible light, which have a higher level of energy, to infrared light, which is lower energy. A solar cell uses a semiconducting material like silicon to absorb the sun’s light and convert it to electrical power. A semiconductor has a special property called a bandgap that allows it to both absorb light and extract the energy from the light as electricity.
Most solar panels have a single absorbing material, such as silicon. There is a tradeoff when choosing the bandgap of the absorbing material. With a smaller bandgap, a wider range of energy from the sun can be absorbed, generating more current. However, a smaller bandgap also means a smaller voltage at which the electrical current can be extracted. Because electrical power is voltage multiplied by current, there is a sweet spot. Too small of a bandgap and the solar cell produces a large current but small voltage and the opposite for too large of a bandgap.
Tandems minimize this tradeoff. When using two absorbers, each absorber specializes in a portion of the solar spectrum rather than a single absorber responsible for the entire solar spectrum. The first absorber is responsible for all visible and ultraviolet particles of light, or photons. Underneath it, the second absorber is responsible for the infrared photons. Having these specialized absorbers minimizes the loss of energy that occurs when sunlight is lost as heat, rather than electric current.
We use the metal-halide perovskite as the first absorber in our tandem to capture the ultraviolet and visible light and silicon as the second absorber to capture the infrared light.
Metal-halide perovskite: specialized absorber
A popular new photovoltaic material is the metal-halide perovskite. The word “perovskite†actually describes the crystalline structure of any material made of three components in a ratio of 1:1:3. The metal-halide perovskite used for photovoltaics is one part metal (commonly lead), three parts halide (commonly iodine), and one part organic molecule (commonly a molecule called methylammonium). When lead, iodine and methylammonium are combined into a perovskite crystal structure, a semiconducting material is formed.

The metal halide perovskite is a rare and exciting material. It works well as a solar cell and specializes in absorbing ultraviolet and visible photons. For a number of reasons, very few materials work well as solar cells and very few of these solar cell materials specialize in this portion of the solar spectrum. This is a major reason that tandem solar cells haven’t been widely used.
It can be formed entirely using processes similar to printing newspapers. The perovskite is dissolved as a solution similar to an ink and is printed onto a glass or silicon substrate, as a newspaper is printed on paper. These processes are very inexpensive. What is perhaps most surprising is that the solar cell works well when made in this fashion. Most solar cell technologies that work well require expensive or specialized processes and tools.
Prototyping tandems
In our experiments, we developed two layers unique to a tandem solar cell that aren’t used in conventional silicon solar cells. We made an electrically connecting layer called a tunnel junction out of silicon that connects the two light-absorbing materials together.
We also made a transparent electrode, which conducts electricity while also letting light pass through it, to connect the solar cell to external wires so that power can be extracted. We made this transparent electrode out of a mesh of silver nanowires, which is similar to a chain link fence made of wires one thousand times thinner than the width of a human hair. With those layers, we can begin to design the other layers in a multi-layered solar cell.
The design of the light-absorbing materials in a tandem is significantly different from standard solar cells. While there is much work to be done, these tandem solar cells made from silicon and metal-halide perovskite hold substantial promise for continuing the evolution of the solar industry.
![]()
Colin Bailie, PhD Candidate and Research Assistant, Stanford University
This article was originally published on The Conversation. Read the original article.









